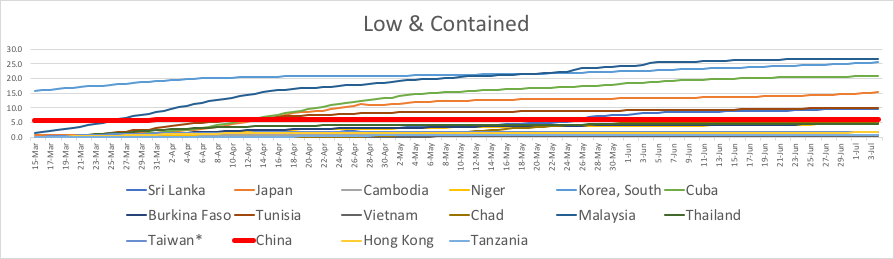
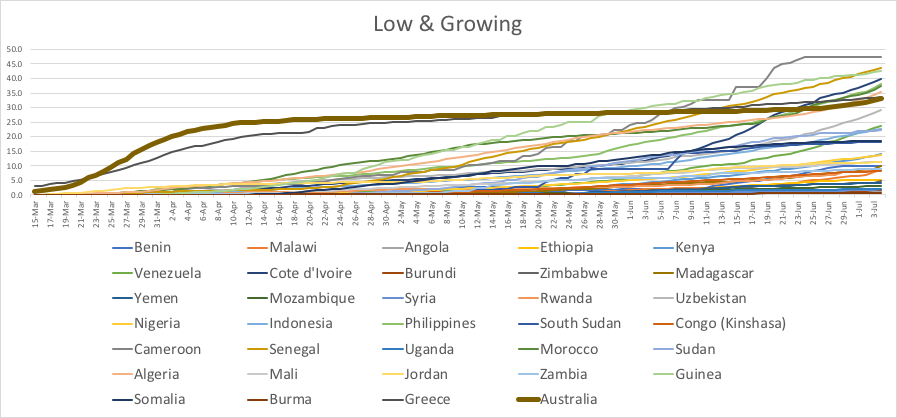
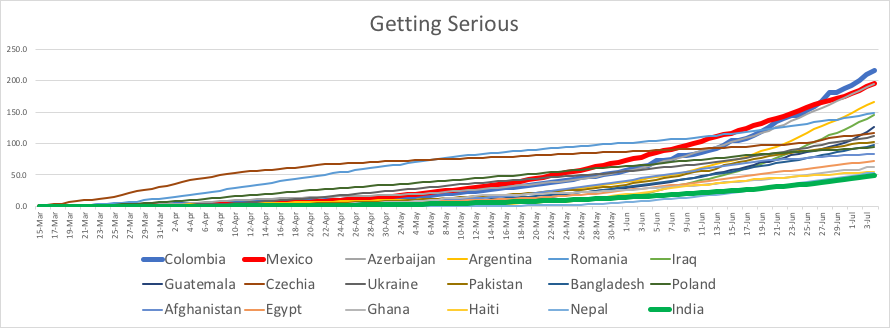
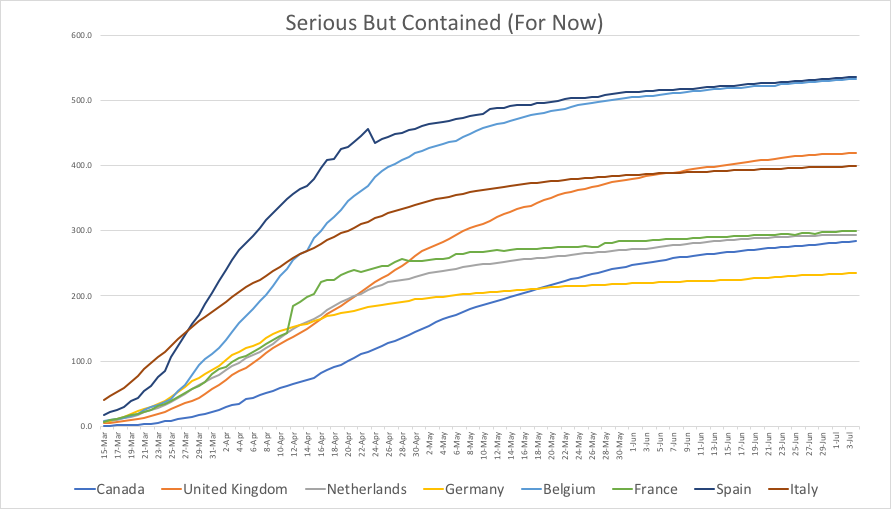
A couple weeks ago, Cornell University had over 80 active infections, and it was looking pretty dire. Then, the students got scared, and more careful. Last week there were 29 new cases. This week only 5. Someone was hospitalized for a few days last week, but it’s down to zero again. Still no local deaths.
Cornell has already run about 90,000 PCR tests since classes started. Students are tested twice weekly, and staff once. It’s probably not cheap. But, there’s a lot to be said for testing everyone: it nips outbreaks in the bud, and also acts as a reminder that the situation is serious. Having same-day turnaround also helps.
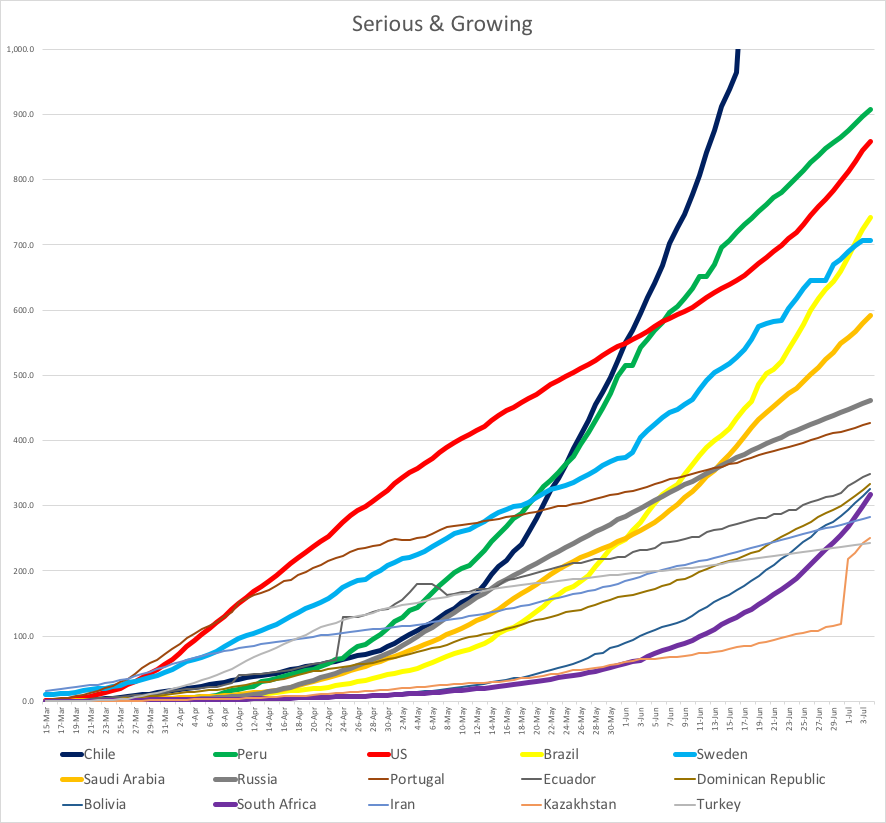
Meanwhile, I’m still doing research for a Coronavirus risk estimator. The consensus now seems to be that most infections happen through the air, so I’ve read a few dozen research publications about droplets and aerosols. The best is a 1934 paper by W. F. Wells. Here are a couple of its charts:

The first shows what happens to droplets that leave a person from talking, coughing or sneezing. Anything smaller than about 140 microns (.14 mm) evaporates, and turns into a floating aerosol. Anything bigger falls to the ground within a few seconds. What that means for Covid-19 is that there are three basic risks:
- if you are close enough to someone, you may inhale one of those bigger droplets while it’s still falling. That’s what the 6-foot rule is all about, and the advice to cough into your elbow.
- After the big droplets land, you can touch that surface, then transfer virus into your eyes, nose or mouth. It’s the reason for washing hands, and not touching your face.
- If you breathe air, you may inhale those small dried-up droplets, which gradually mix into the entire room volume. This is where HVAC comes in. The risk for any space depends on the number of infected people inside and what they are doing, minus air changes and filtration. It’s also why masks are so effective: they block droplets both coming and going.

The second chart from the Wells paper explains why respiratory diseases are more common in winter. People are indoors more, which is half the problem. Even worse, the air is heated and dry, so more droplets evaporate, float around, and end up in noses and lungs.
When working with droplets and aerosols, it’s easiest to do everything in microns (symbol µm, aka micrometers). A micron is one thousandth of a millimeter. It’s about the size of the biggest tobacco smoke particles, a medium-sized bacteria, or the smallest pollen grains. PM2.5 pollution is 2.5 microns and smaller (the most dangerous size because it gets into your lungs easily). N95 masks filter 95% at 1/3 micron size. They hit 99% for both smaller and larger particles. 3M says that’s because the bigger particles are heavy, and ram into a fiber. Smaller ones are extremely light, so the fibers suck them in by electrostatic attraction. 1/3 micron is the sour spot in between. A coronavirus is about 1/10 of a micron.
A few researchers have measured the amount of Coronavirus in mucus and saliva: results range from 12 million to 36 billion virions per cc. It’s simple math to translate that to the amount of virus in droplets of different sizes. As it turns out, a one-micron exhaled particle only has a 2% chance of containing a virus, even at the maximum rate. The bigger droplets that dry up and then float are worse. The maximum size that evaporates in humid air (97 microns) will contain somewhere between 3 and 17,000 virions. The maximum in dry air (172 microns) has 16 to 96,000.
As those droplets lose water, they shrink down to roughly 10 microns diameter (about average pollen size). They become a tiny glob of mucus proteins and passengers, light enough to float for hours, easy to inhale. By the math, those are probably the most dangerous.
Dennis Kolva
Programming Director
TurtleSoft.com